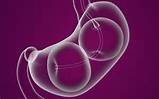
What is "Reshape Integrated Dual Balloon System"?:
Important ReShape™ Integrated Dual Balloon System Safety Information
Indications: The ReShape Integrated Dual Balloon System is indicated for weight reduction when used in conjunction with diet and exercise, in obese patients with a Body Mass Index (BMI) of 30 – 40 kg/m2 and one or more obesity-related comorbid conditions. It is indicated for use in adult patients who have failed weight reduction with diet and exercise alone.
Contraindications: The ReShape Integrated Dual Balloon System is not recommended for patients with conditions that may increase the risk of poor results (e.g., prior gastrointestinal surgery with sequelae, prior open or laparoscopic bariatric surgery, inflammatory diseases of the GI tract, potential upper GI bleeding), who are unwilling to participate in an established medically-supervised diet and behavior modification program, who have alcohol or drug addictions, who receive daily prescribed treatment with aspirin, anti-inflammatory agents, anticoagulants or other gastric irritants, or who currently are or may be pregnant or breast-feeding.
Warnings: The maximum placement period for the ReShape Integrated Dual Balloon is 6 months. The risk of intragastric balloon deflation and intestinal obstruction (and therefore possible complications related to intestinal obstruction) is significantly higher when balloons are left in place longer than 6 months. The presence of blue-green urine or sudden loss of satiety, increased hunger and/or weight gain may be a sign of balloon deflation. Failure of patients to take prescribed daily proton-pump inhibitor medication increases the risk of gastric ulceration or perforation.
Adverse Events: Placement of the ReShapeIntegrated Dual Balloon requires an endoscopic procedure with sedation. Potential risks associated with an endoscopic procedure and sedation include adverse reaction to sedation (headache, muscle pain, nausea), infection, pneumonia, and respiratory distress. Potential risks associated with the ReShape Integrated Dual Balloon include ulceration, perforation, abdominal pain, nausea, vomiting, bloating, belching, heartburn, dehydration, and sore throat. These complications may be severe enough to require early removal of the ReShapeIntegrated Dual Balloon. Although the ReShapeDual Balloon design provides an anti-migration feature, there is the potential risk of device migration and intestinal obstruction. The risk of intestinal obstruction is increased if the device is not removed after 6 months. If intestinal migration occurs, surgical or endoscopic removal may be required.



No comments:
Post a Comment